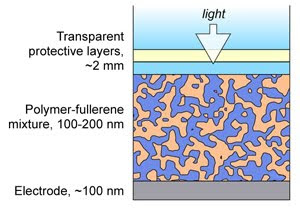
Organic photovoltaics, which rely on organic molecules to capture sunlight and convert it into electricity, are a hot research area because in principle they have significant advantages over traditional rigid silicon cells. Organic photovoltaics start out as a kind of ink that can be applied to flexible surfaces to create solar cell modules that can be spread over large areas as easily as unrolling a carpet. They’d be much cheaper to make and easier to adapt to a wide variety of power applications, but their market share will be limited until the technology improves. Even the best organic photovoltaics convert less than 6 percent of light into electricity and last only a few thousand hours. “The industry believes that if these cells can exceed 10 percent efficiency and 10,000 hours of life, technology adoption will really accelerate,” says NIST’s David Germack. “But to improve them, there is critical need to identify what’s happening in the material, and at this point, we’re only at the beginning.”
The NIST team has advanced that understanding with their latest effort, which provides a powerful new measurement strategy for organic photovoltaics that reveals ways to control how they form. In the most common class of organic photovoltaics, the “ink” is a blend of a polymer that absorbs sunlight, enabling it to give up its electrons, and ball-shaped carbon molecules called fullerenes that collect electrons. When the ink is applied to a surface, the blend hardens into a film that contains a haphazard network of polymers intermixed with fullerene channels. In conventional devices, the polymer network should ideally all reach the bottom of the film while the fullerene channels should ideally all reach the top, so that electricity can flow in the correct direction out of the device. However, if barriers of fullerenes form between the polymers and the bottom edge of the film, the cell’s efficiency will be reduced.
By applying X-ray absorption measurements to the film interfaces, the team discovered that by changing the nature of the electrode surface, it will repulse fullerenes (like oil repulses water) while attracting the polymer. The electrical properties of the interface also change dramatically. The resultant structure gives the light-generated photocurrent more opportunities to reach the proper electrodes and reduces the accumulation of fullerenes at the film bottom, both of which could improve the photovoltaic’s efficiency or lifetime.
“We’ve identified some key parameters needed to optimize what happens at both edges of the film, which means the industry will have a strategy to optimize the cell’s overall performance,” Germack says. “Right now, we’re building on what we’ve learned about the edges to identify what happens throughout the film. This knowledge is really important to help industry figure out how organic cells perform and age so that their life spans will be extended.”
0 comments:
Post a Comment